 STERIS University
STERIS University
20 Courses

Cleaning and Decontamination
An Introduction to Cleaning - Study Guide
All reusable devices must be rendered safe for patient use by performing essential steps in the processing cycle. Cleaning is the most critical component in the process, but residual soil found on device surfaces continues to be a very serious problem. Despite their complexity or their use, reusable devices must be safely reprocessed before being used on another patient.
Course Objectives:
- Define the importance of cleaning reusable devices in clinical practice.
- Describe how cleaning is performed during the reprocessing cycle and how it can be tested.
Cleaning and Decontamination
Optimizing Cleaning Efficiencies - Study Guide
The Sterile Processing department (SPD) has not been immune to the changing health care landscape, often needing to do more, and do it better, with less. This self-paced study guide introduces Lean methods, automation, and cleaning technologies used to optimize cleaning efficiencies and relieve the strain from staff shortages, tighter budgets, and aging equipment.
Objectives:
• Implement Lean principles for process improvement in decontamination
• List methods that help improve cleaning efficiencies

Cleaning and Decontamination
Containers - The Forgotten Medical Device - Webinar
Rigid container systems allow easy assembly of medical devices, but did you know that they are considered medical devices too? Find what you may be missing when processing these critical devices in your department.
Course Objectives:
- List the standards for container reprocessing
- Define the reprocessing process of container systems
- Identify critical inspection points and tools
Cleaning and Decontamination
Critical Contact Points between OR and SPD - Point of Use - Webinar
The Operating Room and Sterile Processing departments have equally important roles for ensuring instruments are properly cared for. While they work closely, the impact that each of their roles has on one another is not well communicated. This one-hour webinar will detail the critical contact points and impact of each department with a specific focus on point of use care of surgical instruments. By discussing expectations, best practices, and identifying cross-departmental consequences of inadequate point of use care, learners will be able to implement successful changes at their facilities to improve SPD and OR point of use care of surgical instruments.
Objectives:
- Identify cross-departmental consequences of SPD and OR actions at point of use
- Discuss expectations, best practices, and identifying cross-departmental consequences of inadequate point of use care
- Discuss critical communication touchpoints related to point of use instrument care for SPD and OR.
- Describe best practices for SPD and OR that encourage success at the point of use.
Cleaning and Decontamination
Decontamination and Safe Handling of Reusable Devices - Webinar
The complexity of reusable device decontamination is a deciding factor in patient outcomes. From the smallest action of hand washing to the complex process of sterilization, every step must be completed correctly. Join Clinical Education Specialist, Sharon Lashley, as she highlights many of the critical and complex processes of decontamination and safe handling of reusable devices.
Objectives:
- Define the critical aspects and steps of decontamination of reusable medical devices
- Describe the AAMI and OSHA recommendations for the decontamination area focusing on employee safety

Cleaning and Decontamination
Recognizing and preventing device damage from water impurities - Webinar
Spots, stains, and rust are common instrument damage caused from water impurities. Join Senior Clinical Education Specialists, Michele McKinley and Pamela Carter, as they unravel the mystery of device damage to discover what caused it and how to prevent it from happening again.
Objectives:
- Identify instrument damage caused by water impurities and the means to prevent it
- Describe methods to distinguish between residual soils and the effect of water impurities
- Recognize the sterile processing technician's role in preserving water quality

Cleaning and Decontamination
Guide to Ultrasonic Cleaning - Study Guide
Many of today’s complex instruments have difficult to clean crevices and lumens. Ultrasonic cleaning technologies supply the power needed to remove residual soils from these difficult-to-clean areas. Correctly performing ultrasonic cleaning requires a knowledge of the equipment, cleaning chemistries, and techniques to maximize cavitation and effectively remove soils.
Course Objectives:
- Describe how ultrasonic cleaning works
- List factors that hinder or help ultrasonic cleaning
- Define safety considerations when operating ultrasonic cleaners

Cleaning and Decontamination
Introduction to ANSI/AAMI ST108 - Webinar
Water is used to clean, disinfect, and sterilize millions of medical devices each day. Many assume that the water from the tap is clean enough for these critical applications. Discover how clean water should be and water’s dirty little secrets as Sr. Clinical Education Specialist, Chasity Seymour, and Clinical Education Specialist, Cody Troutt, introduce water quality and its application to medical device processing through the standard ANSI/AAMI ST108.
Objectives:
· Describe the scope of the ANSI/AAMI ST108 standard, Water for the processing of medical devices
· Describe the focus of each section within the standard

Cleaning and Decontamination
Is it Clean? - Webinar
This instrument looks clean but is it really? Join Tamara as she talks about hidden residual soils and the types of tests that can reveal them.
Objectives:
- List soils found on medical devices
- Identify cleaning tests as defined in ANSI/AAMI ST79
- Establish a quality program for cleaning verification

Cleaning and Decontamination
Look Behind the Curtain of Chemistry Performance - Webinar
Walking down the shampoo aisle and knowing the difference between each kind can be confusing. Some promote curls, others straighten – but in a way, they all look the same. Cleaning chemistries for sterile processing can be equally confusing, but there’s more than just a bad hair day on the line when selecting a cleaning chemistry for medical devices. Join Nancy Kaiser, Director of Science and Technology as we peek behind the curtain of chemistry performance and reveal that there so much more to what’s inside the bottle than you may think.

Cleaning and Decontamination
Manual Cleaning of Instruments - Study Guide
Manual cleaning is as complex as the medical devices. Getting manual cleaning right is a combination of right technique, right chemistries, and right tools. Walk through the steps of manual cleaning and, among other tips, you will discover the impact of point-of-use treatment, what makes a good cleaning chemistry, and how to prevent instrument damage.
Course Objectives:
- Describe the essential elements necessary to ensure optimal outcomes of manual cleaning.
- List the common causes of instrument deterioration/corrosion

Cleaning and Decontamination
Manual Cleaning: Why? How? What if? - Webinar
Proper brush selection is critical to the thorough and effective cleaning of your instruments. This presentation will discuss the different brush materials available and their characteristics and will also explain topics such as antimicrobial properties of brushes, considerations when selecting a brush, differences between disposable & reusable brushes and cleaning and sterilization of brushes. Attendees will also learn how to apply industry guidelines and discuss the risk of not following recommended practices and manufacturers IFUs when using brushes.
Objectives:
Discuss the different brush materials available and their characteristics
Explain antimicrobial properties of brushes
Discuss the considerations when selecting a brush
Explain the difference between disposable & reusable brushes
Apply industry guidelines to all the above objectives
Discuss the risk of not following recommended practices and manufacturer’s IFU when using brushes

Cleaning and Decontamination
Point-of-Use Treatment: Where Successful Processing Begins - Webinar
Point-of-Use treatment is the foundation for successful cleaning. New procedural demands and intricate medical device designs have driven the ever increasing complexity of Point-of-Use treatment.
Objectives:
- List the impact of ineffective point-of-use treatment
- Define the key practices to remove gross soil during Point of Use Treatment
- Describe effective preparation techniques to ensure surface moisture retention and device protection at the Point of Use Treatment

Cleaning and Decontamination
Robotics - A New Age Procedure with Old Age Processing Challenges - Webinar
Robotic-assisted surgeries ushered in a new age of patient care with promises of increased surgeon dexterity, reduce stays, and lower surgeon strain. This new-age equipment suffered an old age problem, tough cleaning. Join Chasity and Tamara as they discuss the challenges and solutions for cleaning robotic-assisted surgery devices in your sterile processing department (SPD).
Upon completion of this training, you will be able to:
- Identify the current trends of robotic surgery and its impact on sterile processing
- Evaluate risks in the processing of robotic surgical instruments
- Establish optimal processing of robotic surgical instruments

Cleaning and Decontamination
The Essentials of Instrument Decontamination - Study Guide
Today, healthcare professionals responsible for instrument preparation and processing are often confronted with a variety of issues involving infection control and decontamination. Complex instrument designs, virulent pathogenic microorganisms, guideline compliance and ongoing educational needs are challenging processing personnel. By understanding infection transmission, the importance of thorough decontamination, and principles of infection control and processing practices, healthcare professionals can develop and implement policies and procedures that will minimize or prevent the spread of potentially infectious microorganisms.
Course Objectives:
- Describe the decontamination environment necessary to prevent disease transmission.
- Review the critical steps of decontamination of reusable medical devices.

Cleaning and Decontamination
The Importance of Surface Disinfection and Departmental Housekeeping - Webinar
The road to cross-contamination is paved with surfaces. Healthcare surfaces become contaminated with microbes from patients, staff, visitors, and vendors. Some of these can have the ability to kill. Wipe out your surface microbial threat using standards, recommended practices and methods proven to be effective.
Course Objectives:
- Review established standards and recommended practice for surface disinfection
- Discuss methods for appropriate departmental housekeeping based on standards

Cleaning and Decontamination
The Principles of Cleaning and Decontamination of Medical Devices - eLearning
Cleaning and decontaminating medical devices is a complex, two-step process beginning with cleaning and ending with a microbiocidal step. Whether a large facility with a lot of automated equipment or a smaller one relying on manual processes, the goal of safe handling must be the same for all. Follow Max as he guides you through the principles of cleaning and decontamination of medical devices.
Course Objectives:
- Define Cleaning & Decontamination
- Understand the different methods of cleaning and decontamination
- Apply the principles behind each method
- Relate various guidelines and recommended practices that play a support role in the decontamination process and why

Cleaning and Decontamination
The Science, Equipment, and Methods of Cleaning - Webinar
Safe medical instrumentation starts with effective cleaning. Learn the secrets to manual and automated cleaning of reusable medical devices.
Objectives:
• Define clean
• List 4 factors that influence cleaning efficacy
• Describe the methods and equipment used to clean medical devices
Cleaning and Decontamination
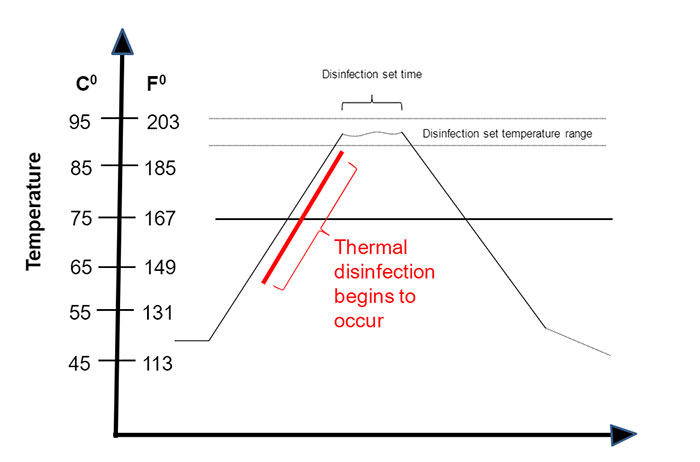
Understanding Thermal Disinfection Using A0 - Webinar
A0 ("A-Sub-Zero") is an expression of the time required to achieve a specified log reduction of microorganisms based on the temperature of the thermal disinfection cycle. This course will provide you with a basic understanding of thermal disinfection and answer the question "What is A0?".
Course Objectives:
- Define, and review the basic principles of A0 ("A-Sub-Zero")
- Identify the differences and requirements for thermal disinfection in the United States and Europe.

Cleaning and Decontamination
Water Quality for Processing Medical Devices - Study Guide
It is important for any department that reprocesses and utilizes medical instrumentation and devices to be able to identify potential problems with water quality and initiate the measures necessary to ensure safety for patients and instrumentation. Ensuring the recommended water quality for the processing of medical devices requires collaboration and communication between processing staff and those who establish and maintain the water treatment program.
Learning Objectives:
• Identify the types and causes of instrument damage from water impurities that can impact patient outcomes
• Name the levels of water quality necessary for specific steps in device cleaning, rinsing, high level disinfection, and steam sterilization
• Describe the types of water treatment systems used in device processing
