 STERIS University
STERIS University
21 Courses
Leadership & Management
Sterilization Failures - How They Fit Into Your QMS Program- Webinar
Why wait for a sterilization failure to happen to improve sterilization processes? Quality management systems (QMS) provides proven methods to find and treat potential problems and sterilization failure. This program will show how sterilization failures fit into your department’s QMS.
Course Objectives:
- Identify sterilization failures
- Identify preventive actions to prevent sterilization failures
- Identify how sterilization failures fit into the departmental Quality Management System (QMS) program

Leadership & Management
A 4-Step Process for an Effective Water Treatment System- Webinar
Water quality plays a “critical” role in medical device processing. Ensuring that quality requires proper design and maintenance of an effective water treatment system. Join Brian Battani, Business Development Manager for Xylem as he shares the four steps to an effective SPD water treatment system.
Objectives:
• Describe four steps to obtain ensure an effective water treatment system
• List typical quality control measures used for water treatment systems
• State required maintenance necessary for peak water treatment system performance

Leadership & Management
A Systematic Approach to Replace Hospital Ethylene Oxide - Study Guide
Moving away from ethylene oxide sterilization in sterile processing may seem complicated and overwhelming. Choosing a different process, confirming devices can be sterilized, safety, training, installation, and quality assurance are just a few considerations. How should a department supervisor move forward with the change? Using a systematic approach to evaluate options and manage the change process can simplify and ease the switch.
Course Objectives:
- Identify important considerations when evaluating a new low temperature sterilization process
- Prepare for implementation of a low temperature sterilization process
Leadership & Management
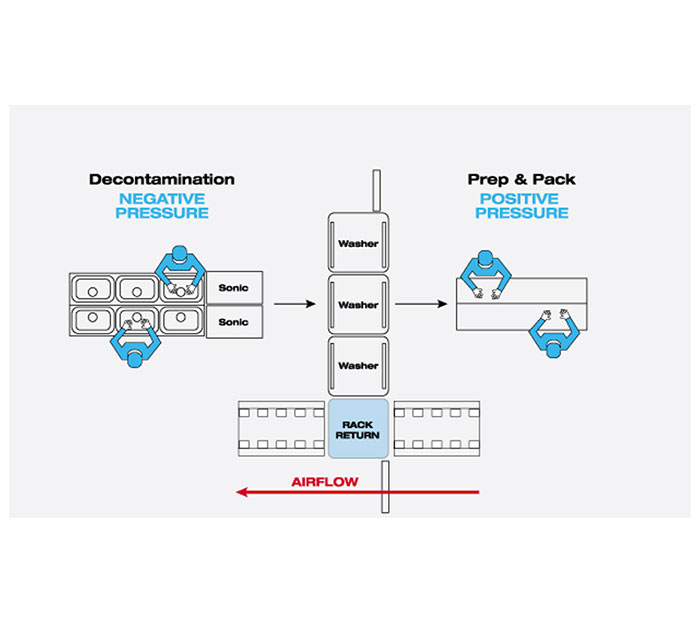
Air Management - What Does It Mean to SPD?- Webinar
Are you positive about your positive pressure rooms? Join Lisa Huber BA, CRCST, CIS, CHL, ACE, FCS, CQIA (Clinical Education Specialist – Midwest Region with STERIS) as she reviews the equipment, processes, and guidelines needed to control the air pressure in your Sterile Processing Department.
Course Objectives:
- Match air pressure requirements to facility rooms
- Identify the components within the air management system
- List ways to manage air pressure within SPD
Leadership & Management
Are You Ready To Attract the New Workforce?- Webinar
With a shrinking workforce, how do you fill open sterile processing positions? By creating a workplace that employees want to be. Join our panel of experts as they discuss how workforce needs are changing and tactics you need to hire and keep sterile processing personnel.
Objectives:
- List similarities and differences between the traditional and non-traditional workforce
- Describe three management activities that build a technician focused department
- Articulate methods to implement workplace flexibility, create a culture of safety, and improve work expectations within sterile processing

Leadership & Management
Optimize Utilities to Optimize your Sterile Processing Equipment Performance - Webinar
Learn the importance of maintaining water and steam utility integrity to prevent sterilizer downtime and ensure that infection prevention technology remains fully operational. Join our experts as they share valuable tips and tricks to proactively address your utilities, ensuring smooth and efficient operations in your Sterile Processing Department (SPD).
Objectives:
- List the types of sterile processing equipment shutdowns and the impact on operations
- Describe measures that can be taken to improve sterile processing utilities
- Discuss challenges when incorporating utility standards and methods to maintain compliance

Leadership & Management
Collaboration Learning - Social Media, Friend or Foe of the Healthcare System- Webinar
Join Pamela for a free CE webinar to learn training and education techniques and how technology can help you develop a successful learning program.
Course Objectives:
- Identify historical training and education techniques
- Understand how new technology can assist in ongoing education and learning
- Identify challenges moving into a more technology-based learning platform
- Identify tips on how to navigate and develop a successful learning program in the social media age

Leadership & Management
Emergency Preparedness and Safety In Sterile Processing Departments - Webinar
Emergencies by nature are unpredictable, but rapid, well organized emergency response is crucial for patient and employee safety. Join Stephanie Boroz, Associate Director of Sterile Processing at Wyoming Medical Center as she investigates the risks associated with day-to-day processes, equipping staff with the necessary knowledge and tools, and fostering a culture of safety.
Objectives:
- Recognizing the risks within sterile processing.
- Prevention and mitigation of environmental hazards.
- Responding to chemical spills.
- Managing exposure to bloodborne pathogens.
- Response to event, resource, and equipment disruption.
- Recovery and stabilization efforts

Leadership & Management
Establishing a Vision for Professional Growth- Webinar
Objectives:
• Ascertain desirable characteristics of typical sterile processing professional positions
• Develop a plan for professional growth
• List resources that can help with professional growth

Leadership & Management
Keeping Your House Clean - Sprucing Up Your Instrument Tracking Database- Webinar
Are you interested in cutting the clutter and tidying up your sterile processing tracking system or instrument and set database? Profit from the benefits of a well-managed, clean database. Route out redundancies, inaccuracies, and other data fallacies with reports that make it easy. Include everyday management techniques to keep it clean.
Course Objectives:
- Understand the attributes of a clean database
- Identify the four rules of master database management
- Recognize the types of reports that are most helpful for data clean

Leadership & Management
Lean Measurements You Can Brag About- Webinar
Objectives:
• Describe the key principles of a Lean SPD.
• List the key measurements used to monitor lean SPD progress.
• Implement lean measurements.

Leadership & Management
Leveraging Service Technology for Operational Efficiency and Staff Satisfaction - Webinar
Keeping sterile processing equipment running is the goal of all facilities, biomeds, and sterile processing leaders but did you know records from maintenance, repairs, and quality assurance results are part of a comprehensive survey? These distinct departments must work together to ensure the documentation is retrievable and complete. Let Andrey Tsupruk walk you through the many ways this can be accomplished in the digital age.
Objectives:
- List service documentation requirements for a successful accreditation survey.
- Compare and contrast service documentation retention methods used in healthcare facilities
- Leveraging technology to improve equipment servicing efficiencies

Leadership & Management
Loaned Instrument Tray Management - A Shared Responsibility- Webinar
The use of loaned instrumentation varies by each facility, but these days everyone uses loaned trays of some sort, at some time. Proper management and processing of these instruments is critical to patient safety.
Course Objectives:
- Identify the impact on patient safety when loaner trays are mismanaged and improperly reprocessed.
- Discuss HSPA’s loaner policy and documents available to support implementing a structured process.
Leadership & Management
Make Tracking Software Your Workflow Partner- Webinar
Sterilized, complete, and on time...easier said than done. Every sterile processing supervisor/manager strives to reach and maintain this goal, but do you know how consistently it is reached, or areas for improvement? Join Mark Capel (Chief Digital Officer with STERIS) as he translates the data found in workflow systems into actionable quality improvement initiatives.
Course Objectives:
- List ways that workflow software helps SPD respond in real-time for the unexpected needs of the operating room
- Identify key workflow features that support technician competency
- Develop reports and tools to improve Safety, Quality, Delivery, and Cost

Leadership & Management
Process Management and Control in a Healthcare Operation - Study Guide
The daily journey of navigating the department's processes and meeting Customer requirements would be daunting if not for quality systems, specifically, quality management systems. A quality management system (QMS) can be viewed as the department's foundation and framework to include instructions, procedures, and resources to support quality management. Over the years, quality systems have matured into applications seeking transparency, reproducibility as well as Customer satisfaction as a result of systematic thinking, auditing and documentation. Today, health care facilities are credentialed by a number of organizations under the watchful eye of the Center for Medicaid and Medicare Services (CMS). These organizations will review a health care facility's documentation for completeness and observe the processes for which procedures and instructions are documented.

Leadership & Management
QMS for the SPD- Webinar
It’s
another phone call. It’s not the OR yelling about a missing instrument. It’s
not a late loaned instrument set. This time it’s the audit room. The auditor just
asked about a missing biological indicator test result. All three calls may
have been avoided with a good quality management system (QMS), but do you know
what a QMS is? Let us introduce you to Quality Management Systems for the
Sterile Processing Department.
Objectives:
- List the components of a quality management system
- Identify elements specific for a sterile processing quality management system
- Incorporate standards, guidance, and regulations into quality management systems
Leadership & Management
The Challenges of Prion Decontamination- eLearning
Prions are the protein-based, transmissible causative agents of a group of diseases known as transmissible spongiform encephalopathies (or prion diseases). Prion diseases present a unique risk because although they are not considered contagious they can be transmitted between patients via tissues and reusable medical device surfaces because prions are highly resistant to decontamination. Understanding these diseases is important in the development of facility policies to reduce patient and staff risks. Therefore, it is imperative the Sterile Processing Department (SPD), Operating Room and other department’s supporting clinical and surgical interventions understand these diseases when developing policy and procedures for the use and reprocessing of medical devices.
Course Objectives:
- Introduction to the prions and prion diseases
- Explain the risks associated with prion decontamination
- Review what steps a facility should take to review the risk of prion transmission on reusable devices"
Leadership & Management
The New Frontier: Hospital Sterilization of 3D Printed Devices - Webinar
Healthcare has entered a new frontier of patient care with 3D printed medical devices. With improved patient satisfaction and outcomes, 3D printed Medical Devices are spreading like wildfire across the country. Learn what you should be doing to prepare for sterilization of 3D printed medical devices in your sterile processing department.
Objectives:
- Identify what 3D printing of medical devices is
- Identify challenges of processing 3D printed devices
- Identify steps in processing of 3D printed devices
Leadership & Management
The WHY behind WHAT we do - A Focus on Quality in SPD- Webinar
Seeing a tidal wave of case carts. Listening to complaints from the OR. Rushing to process devices. It’s easy to forget the real reason sterile processing exists: The Patient. Join, Director of Clinical Education with STERIS, Karen Owens , as she reminds us of the WHY behind WHAT we do and how sterile processing makes a difference to the Patient.
Objectives for this course are:
- Identify the “why” behind the role of sterile processing in patient care
- Discuss key practices that help sterile processing provide quality products to customers
- Explain the direct connection between quality processes in sterile processing and excellent patient outcomes

Leadership & Management
Water. The Life's Blood of Sterile Processing- Webinar
Water is used in every application of medical device processing. Without it, processing stops. You could say, it is the life’s blood of the processing department. Just as routine blood tests capture health problems, water tests capture quality issues that lead to processing problems. Let, Sr Principal Scientist, Herb Kaiser, show you the routine water tests that find the processing problems lurking in your water.
Objectives:
· List the properties of pure water
· Describe the impact water impurities have on sterile processing activities
· List methods of water testing for impurities of concern

Leadership & Management
Weathering the Storm of Staffing Challenges- Webinar
A storm of staff shortages? It’s more like a hurricane. Finding good staff is one thing, keeping them is another. Join Cody McElroy, Manager of Sterile Processing at University Hospitals Cleveland Medical Center, and Aaron “Cody” Troutt, Director of Central Sterile at Williamson Medical Center, as they discuss ways to engage and retain staff in during this tumultuous time.
Objectives:
- Implementing a meaningful reward system through personal motivators
- Engaging Customers to motivate staff performance
- Mentoring to increase staff performance
- Using tactics to retain high-performing staff members
